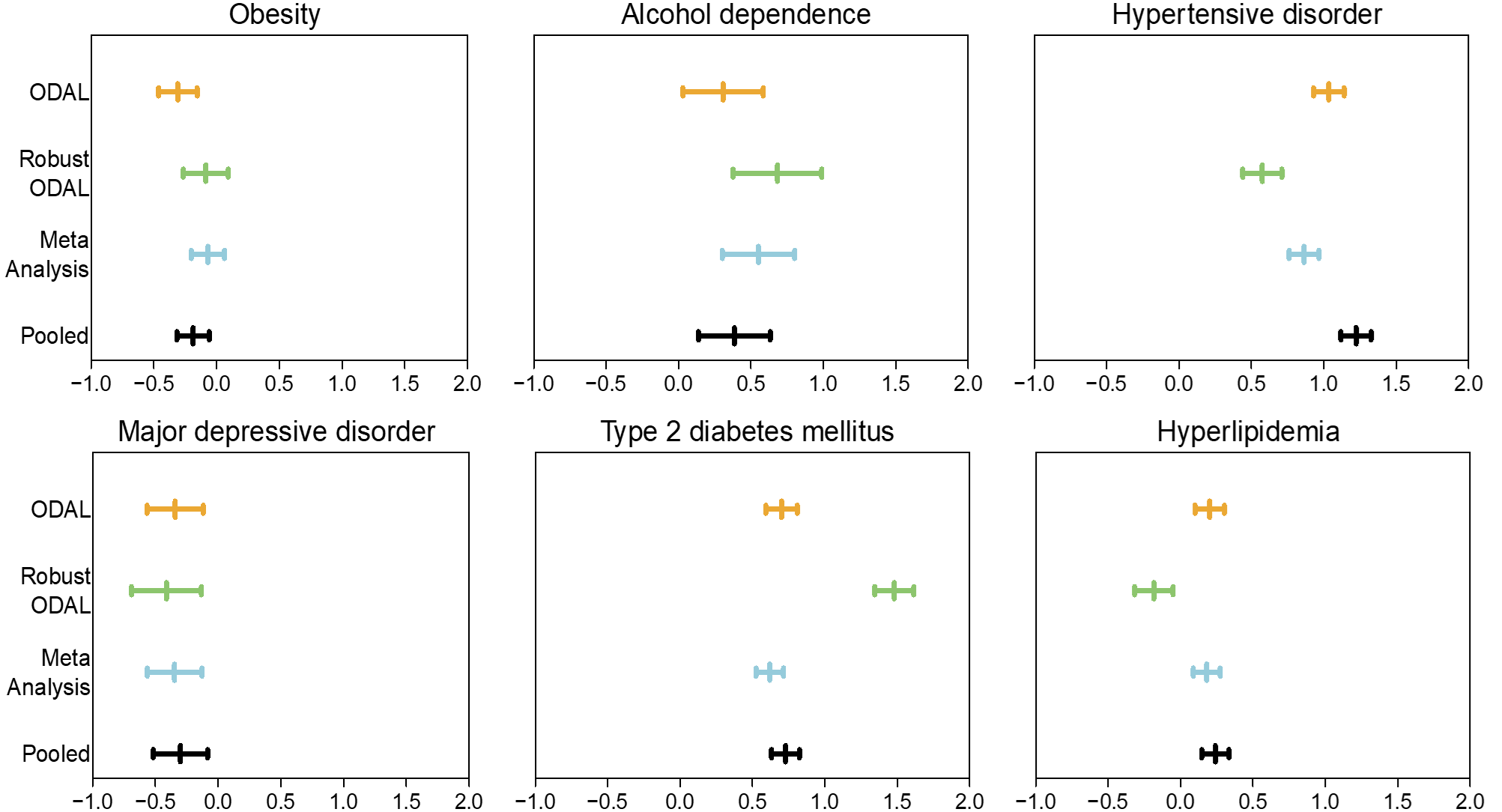
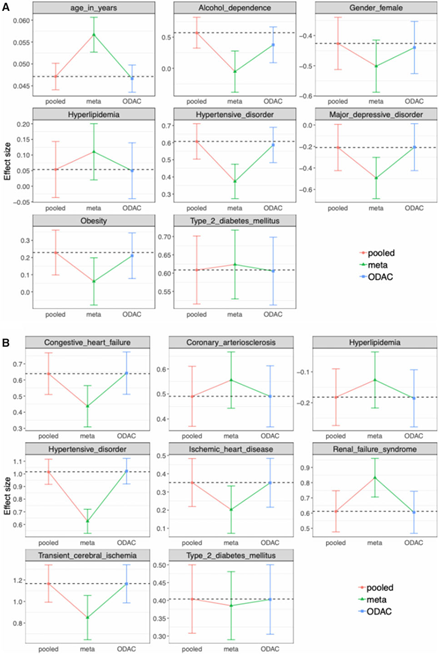
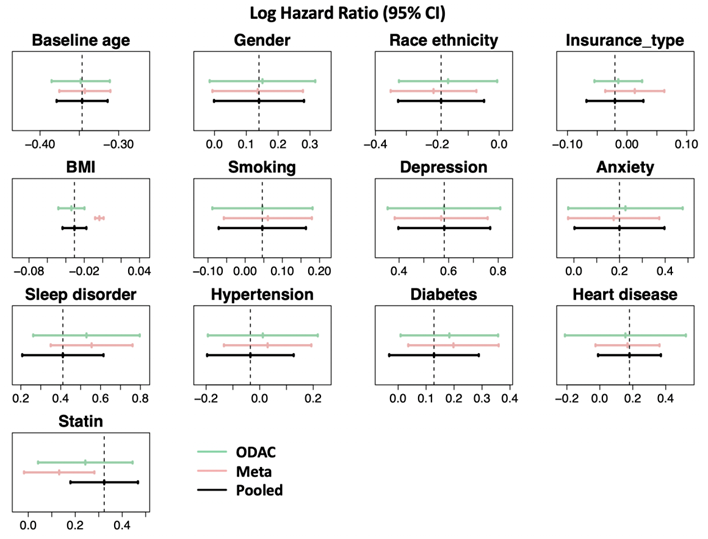
A collabratobe study was conducted invoving five participating sites of OneFlorida to study the association between OUD and several relevant clinical risk factors among individuals receiving at least one opioid prescription.
Each participating site extracted EHR records between 01/01/2012 and 03/01/2019 for patients who had opioid prescription (including Codeine, Fentanyl, Hydromorphone, Meperidine, Methadone, Morphine, Oxycodone, Tramadol, Hydrocodone, Buprenorphine), and no cancer or diagnosis of opioid use disorder before their first prescription. Among these patients who were exposed to opioid, a case of opioid use disorder is defined as having first diagnosis of opioid use disorder within 12 months after their first prescription and a control is defined as having no diagnosis of opioid use disorder in the entire time window.
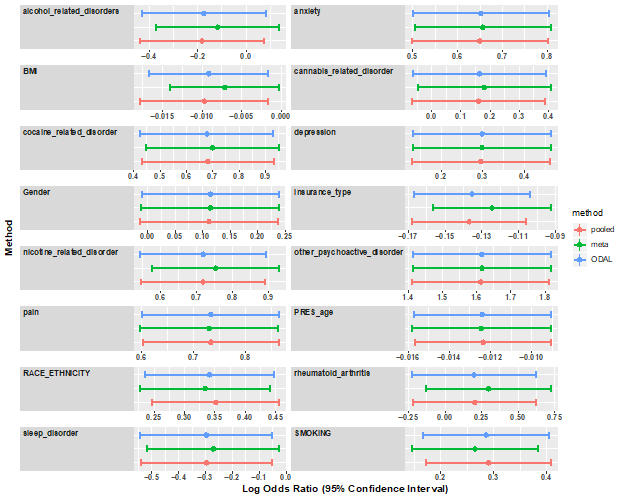
Logistic regression chosen to model the outcome variable and the following risk factors: age, race, gender, and insurance type, Race/ethnicity, and having at least one diagnosis of anxiety, alcohol use disorders, depression, sleep disorders, rheumatoid arthritis, other pain conditions, cannabis-related disorders, nicotine-related disorders, other psychoactive disorders, cocaine-related disorders.
Estimated odds ratio are shown in the following figure…